Transition Metal-Sulfide Systems-Abiogenic vs Biogenic
Evolution of single metal sulfides
The physicochemical properties of a nanomineral are strongly affected by its formation processes, and thus, may indicate the nanomineral’s formation environment and mechanism. This correlation, although relevant to a myriad of geological, environmental, and material-science processes, has not yet been fully appreciated and systematically explored. In this project, our major focus is to investigate the crystal structure and reactivity of biogenic metal sulfide nanominerals and compare these biogenic nanomineral with their abiogenic counterparts.
Mixed metal cation sulfide systems
It is exceptionally rare to find pure metal sulfides in nature. Most naturally occurring iron-, copper-, zinc-, and lead-sulfide have other metal elements incorporated in their crystal structures. How the co-existence of multiple metal elements may affect the structure and resultant stability/reactivity of metal sulfide phases, particularly those in the nanoscale regime, however, remains, for the most part, a mystery. In this project, we will investigate biogenic versus abiogenic mixed metal sulfide nanominerals, e.g., Fe-Zn, Fe-Cu, and Fe-Co sulfide systems. Our focus will still be these nanominerals' crystal structure, formation mechanisms, and reactivities. (Images below: self-assembly of biogenic ZnS nanocrystals; and transformation of biogenic Fe-sulfide from an amorphous structure to nanorods.)
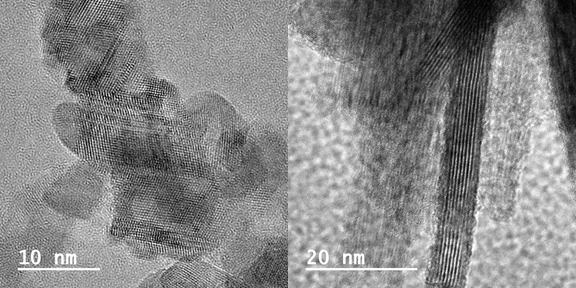
Mixed metal cation-anion-sulfide systems
The emphasis is on the abiotic and biological conditions leading to solid-phase formation and the physicochemical properties of the formed solid phases. The main method used are STEM-EELS, XPS, EXAFS (synchrotron).
Roles of Metal Sulfide Nanostructures in Nature
As natural catalysts
Being developed.
As energy source for anoxygenic photosynthesis
Nanomineral has the potential to greatly affect the behavior of associated microorganisms. For example, the former work of Jie revealed that the oxide nanominerals with similar sizes and shapes showed distinct effects on the survival rate of associated bacterial cells. This work also brought forward the idea of “mineral surface chemistry-directed increase of cell surface complexity” by discovering that more cytotoxic mineral surfaces stimulated the generation of greater amounts of EPS in the bacterial cells to enable community living in biofilms ( Xu et al., 2012 Astrobiology; Xu et al., 2013 EPSL). Illuminating the interactions between nanominerals and microbial cells remains a focus of our NanoGeoBio Laboratory.
Phototrophy-Coupled Microbial Sulfur Cycling
Overview
The coupling of phototrophy with sulfide-oxidation and/or sulfate-reduction provides a plausible mechanism for microorganisms to tackle some of the restricting conditions in gypsum-dominated and sulfidic environments. The major motivation for this project is to illuminate the relationships between bacterial phototrophy, sulfide-oxidation (or sulfur-oxidation), and sulfate-reduction in water-restricted gypsum dunes, and in shallow sulfidic water bodies. We are combining analyses of field samples with lab simulation experiments for this work.
In gypsum dunes

(the White Sands dunes, New Mexico)
In sulfidic settings
Oil contaminated mud flat sites along the Gulf of Mexico. (The copyright of the image belongs to S. Burgess.)

In the Great Salt Lake
Significant abundances of phototrophic sulfur-cyclers have been previously identified in the Great Salt Lake, Utah. Their environmental roles and adaptation to the rapid changing climate remain to be scrutinized however. The lab is collaborating with Dr. Bonnie from the Great Salt Lake Institute, trying to illuminate the roles of phototrophic sulfur-oxidizers and their relationship with other communities in this hypersaline environment.
Detection and Characterization of Nanominerals in Complex Matrices
Systematic characterization
Intricately-structured nanoscale clusters, particles, crystals and aggregates (i.e., nanomaterials) are produced via natural processes in a wide range of geological settings, from groundwater, critical zones, volcanic ashes, glaciers, to lacustrine, riverine, estuarine, and marine sediments. Due to their extremely small sizes and high reactivities, these naturally occurring nanomaterials have the potential to greatly modify their relevant environments and processes. The nature and extent of the interactions of these indigenous nanomaterials with other biogeochemical components is literally terra incognita. For example, metal-sulfide nanoparticles in mud flats may enhance the solar-energy conversion via forming synergies with photosynthetic bacteria, nanoparticles carried by glaciers could mediate the nutrient cycling and ecological balances in polar regions, or the inhalation of nanoparticles through exposure to fly ashes may affect the way human brains work.
A primary reason for this lack of understanding of the roles of naturally occurring nanomaterials is because scientists haven’t established an efficient way to separate them from their heterogeneous and complex natural matrices, thereby leaving us with no quantitative information and little qualitative description for their presence. In this project, Jie along with peer researchers at UTEP intend to develop a high-throughput facility that is specifically designed to isolate and sequentially characterize, both quantitatively and qualitatively, naturally occurring nanomaterials in various geological and environmental samples. This nano-facility will be essential for understanding the types and quantities of nanomaterials occurring in nature, necessary information for deciphering their biogeochemical roles in relevant environments.
Copyright © Jie Xu 2024. Last updated: 2024-12-31 17 36